|
· A Triple Threat!
· ISOTOPE ALERT: AMIC Makes Local Delivery
· PARTNERS BY DESIGN: Partnering on Proton Therapy
A Triple Threat!
GE, Gamma offer preclinical trimodality scanner
Continuing its focus on early health, UK-based GE Healthcare recently announced an agreement with multimodality imaging manufacturer Gamma Medica Ideas, which has US operations based in Northridge, Calif.

|
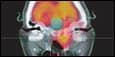
| Trimodality scanners allow researchers to look at a broad range of investigational problems. |
Since July 1, GE has been the exclusive distributor of Gamma Medica-Ideas’ preclinical imaging products, which play a key role in new pharmaceutical therapeutic development. According to GE, the deal gives its researchers greater access to GM-I’s fully digital, trimodality PET/SPECT/CT scanners.
“Preclinical imaging is a bridge between life sciences research for studying disease at the molecular level in cells, to in vivo studies of the disease,” explained Kirill Shalyaev, global marketing manager for GE Molecular Imaging’s technology business. “Before anything goes to clinical practice for human applications, it has to be proven in animal models, and that’s where clinical imaging provides a key role in virtual translation.”
The trimodality scanners allow researchers to look at a broad range of investigational problems, enabling them to monitor several biological and biochemical mechanisms in the middle of the disease simultaneously, Shalyaev said. In the area of SPECT imaging, the scanners are equipped with new CCT-based detector technology, exhibiting high-energy resolution that lets scientists image different radiotracers at the same time.
“This agreement is an opportunity for customers to gain greater access to our hybrid scanners by leveraging the extensive footprint of GE Healthcare,” said Bradley Patt, PhD, president and CEO of GM-I. “We were first to market a trimodal, preclinical scanner, and our all-digital technology provides improved spatial resolution while enabling researchers to share images easily—bringing digital pathology to the preclinical arena.”
Adding to its offerings of dual-modality PET/CT, CZT-based SPECT/CT, and dedicated in vivo and specimen preclinical CT scanners, GE said it looks forward to investigating disease biology and developing future drugs with GM-I, who has installed more than 50 multimodality systems in four continents since 2002.
“This agreement underscores our deep commitment to the molecular imaging and preclinical markets,” said Eugene Saragnese, vice president and general manager, molecular imaging and CT at GE. “Many innovations in clinical imaging get their start in the preclinical devices, and we continue to look to that space as a showcase for what’s possible for the future of human PET, SPECT, and CT imaging.”
Isotope Alert
AMIC Makes Local Delivery
When Kadlec Medical Center of Richland, Wash, required more supply of fluorodeoxyglucose (FDG), the company didn’t need to look very far. Advanced Medical Isotope Corp (AMIC), Kennewick, Wash, was happy to make the delivery.

|
“AMIC is delighted to assist our region’s health care needs and be a local source for the production of a multitude of radioisotopes,” said William J. Stokes, president of AMIC. “We have a committed team of professionals with a passionate mission to deliver high-quality medical isotopes in a timely and cost-efficient manner.”
The medical isotope company produces and distributes medical isotopes and medical isotope in vivo delivery systems for advanced diagnostic and nonsurgical therapeutic environments.
AMIC recently announced the delivery of short-lived radioisotopes to be used in positron emission tomography, which the company says is rapidly becoming the preferred scanning technique in medical research.
FDG is being utilized for a range of purposes, including diagnosing and monitoring the treatment of Hodgkin’s disease, non-Hodgkin’s lymphoma, Alzheimer’s disease, and lung cancer.
To learn more about AMIC’s plans for isotope production in the United States, see our cover story in the September issue of Axis Imaging News. Or read it online at www.imagingeconomics.com.
Partners by Design
Partnering on Proton Therapy
Varian Medical Systems, Palo Alto, Calif, and Still River Systems Inc, Littleton, Mass, recently announced they will partner in designing an interface between Varian’s ARIA Oncology Information System and Still River’s Monarch250 Proton Beam Radiotherapy System, which is in the process of development. In doing so, the pair aims to bring ARIA into the proton environment, helping staff transition from conventional radiotherapy to proton therapy.
“Workflow is critical in both forms of radiotherapy, and given the similarities in the processes, we are working with Still River to ensure continuity,” said James DeFilippi, business manager, Proton Ancillary Business, Varian. “Still River’s proton therapy systems are likely to be added at existing radiation therapy departments, so maintenance of workflow and process consistency will be critical to these sites.”
As a comprehensive information and image-management system, ARIA is a complete oncology EMR that supports a multidisciplinary approach to treatment. It can provide physicians and staff with immediate access to important patient information, helping users to effectively manage treatment plans and influence treatment decisions. DeFilippi said ARIA aids clinicians in coming up with a personalized care plan for each individual patient, from initial diagnosis through follow-up, and it aids authorized users to access lab results, pharmacy/drug orders, and medical oncology information. Physicians also can prescribe treatments, create and edit plans, track dose, and review reference images using ARIA’s treatment plan management functionality.
Useful for both medical and radiation oncology practices, a key component of the ARIA’s process optimization is its CarePath, which simplifies the process of getting patients in the system and onto proven treatment protocols. Together with Dynamic Documents, CarePath assists departments in capturing their daily activities. ARIA also provides evidence-based medicine in an electronic format, which DeFilippi says is “particularly pertinent in the face of pay-for-performance systems.”
“Varian is committed to creating a completely open environment and ensuring connectivity between ARIA and most commonly used radiation delivery devices, including proton treatment systems,” said Corey Zankowski, Varian’s senior director for product management. ARIA can provide up-to-the-minute digital images from a range of modalities, including MV, kV, conventional CT, CBCT, MR, and PET. An Offline Review permits physicians to supervise treatment from a remote location, where they can review images and approve shifts.
Still awaiting FDA clearance for commercial use in clinical therapy, the Monarch250 proton therapy system is integrated with existing clinical systems, including a robotic couch, 2D and 3D IGRT with CBCT, “record and verify,” and treatment planning connectivity. “Its unique and patent-pending design combines current and proven proton therapy technology with modern superconducting magnet technology to reduce the size and cost of the system and potentially improve reliability,” said Lionel Bouchet, PhD, director of product management at Still River.
DeFilippi said the interface will promote an efficient workflow by allowing therapists to work in a manner familiar to them from their experience using a VarianClinac accelerator. “While the Monarch250 system will control delivery of the proton beam to ensure that the patient receives the proper dose during treatment, Varian plans to add an additional verification process within the ARIA system.”
Under the agreement, Still River Systems will provide technical assistance to Varian engineers, who will develop the interface to allow R&V functionality across the two systems.
Any new ARIA interface that facilitates R&V functions with new radiotherapy delivery technologies are subject to FDA clearance prior to any clinical deployment. Still River Systems and Varian expect the development project to take 12 to 18 months, after which it will be subject to FDA approval.




