FDA Approved: Two Mammography Solutions Cleared by the FDA
Kodak Introduces Software for Controlling Dose Creep
Discovery DXA System Approved for Use in Detecting Cardiovascular Disease
Partnership Aims to Increase Detection Rate of Lung Lesions
Running the Numbers
FDA Approved: Two Mammography Solutions Cleared by the FDA
|
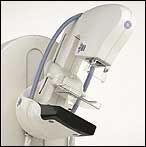
| The Senographe Essential FFDM system from GE Healthcare combines the latest in digital imaging technology with an increased field of view. |
The FDA announced it has approved two new digital mammography solutions in recent months: first, a next-generation digital mammography system from GE Healthcare (Waukesha, Wis), the Senographe Essential; second, the Mammomat Novation(DR) from Siemens Medical Solutions (Malvern, Pa) for mobile use.
The Senographe, first launched in 2000 after 15 years and $200 million in development, was the world’s first full-field digital mammography (FFDM) system. The latest addition to the line, the Senographe Essential, provides the largest active field of view available, with a detector area of 24 x 31 cm.
According to Jennifer Connor, director of breast imaging at GE Healthcare, “[The new Senographe] has the lowest dose in the industry and delivers the highest DQE [detective quantum efficiency] in the market. That’s important, especially as you start to look at 3D imaging. It will make a big difference to be using those types of technologies on this type of platform.”

|
| The Mammomat Novation(DR) from Siemens Medical is now approved for use in mobile applications. |
Next, Siemens Medical’s Mammomat Novation(DR) is now approved to be hooked up to a mobile coach, which offers clinicians improved functionality and flexibility in the mobile environment.
“Digital mammography is quickly becoming the new gold standard for breast-cancer screening and detection,” said Diane Livengood, director of women’s health at Siemens Medical. “Now, our customers can use the Mammomat Novation(DR) in a mobile environment to reach more patients. This is especially important in improving access for women who may have difficulty traveling to an imaging facility that offers digital mammography.”
The Novation(DR) provides digital screening, diagnosis, and stereotactic biopsy capabilities in one system. Additionally, it enables clinicians to better pinpoint disease and plan treatment. With the mobile configuration, medical facilities can bring digital mammography directly to their patients and offer comprehensive women’s health services on the road.
Featuring a flat-panel detector that is based on amorphous selenium (aSe) technology, the Novation(DR) enables a direct conversion of x-ray to digital information. The aSe detector technology has the potential to provide higher spatial resolution and greater clinical detail, which aid clinicians in their diagnosis. Also, at 24 x 29 cm, the system’s image detector enables imaging of a range of patient breast sizes, and its paddle is designed to provide easier and more comfortable patient positioning.
Kodak Introduces Software for Controlling Dose Creep
In the transition from traditional film-based radiology to digital, many steps of the process have been lost or altered. The exposure latitude that’s built into CR systems might help technologists achieve diagnostic quality in difficult exams; however, it also allows for the overexposure of patients.
“The natural tendency is that if you overexpose a little bit, the image not only doesn’t come out bad, it comes out better. The problem is that you can get in a habit of doing it, otherwise known as dose creep,” said Todd Minnigh, national director of marketing for the Americas at Eastman Kodak Co (Rochester, NY). The company has released new software for its CR 800 and 900 series systems that equips administrators with an easy way to monitor repeats and exposures for every exam and every radiographer in a department.
The detailed information from the software provides administrators with an overall picture of their departments. In turn, administrators can provide additional training to prevent the added radiation dose that can occur due to the exposure latitude provided by CR systems.
One of the problems behind the dose-creep habit stems from what initially seemed like a good thing—the impermanence of bad images. “One of the things we did when we came out with digital x-ray was eliminate the film and add a new feature: the delete button,” Minnigh said. “So if I made a picture I didn’t like, I could just delete it. It became a very bad thing, although it didn’t seem that way—it seemed like a digital camera.
“If you’re shooting over or under the range, the CR machine is keeping track—but usually only on a machine-by-machine basis,” he continued. “[With the software], Kodak provides the ability to aggregate all of the data across the department and give the manager, from his or her desktop, a review of the technologist’s performance, irrespective of the machine the tech used or what x-ray room he or she was exposing in. Now, the administrator receives an Excel file of not only the tech’s use of dose—or, in this case, exposure index—but also a report on the repeat analysis. Every time that someone does a repeat or deletes an image, the system will ask why, and then this new software will provide the administrator with an overview of all the repeats in the department and see exactly where the troubles are. That really helps to improve quality.”
Kodak expects to release the software for its CR systems in the near future, although no specific dates have been confirmed. Kodak is aiming to release the software for its DR systems in about 6 months.
“[Dose creep] is a common problem with all digital x-ray systems, and it’s very common in CR,” Minnigh said. “The only way to manage it is centrally by person, because the person is the one making the decision as to how much dose to use—particularly in portables or in places where the technologist has the option to use large-, small-, or medium-patient settings. If they’re always selecting to use a dose for a larger patient, they’re watching their dose creep up, just by the natural tendency to not want to make a mistake.”
—M. Said
Discovery DXA System Approved for Use in Detecting Cardiovascular Disease

|
| Using the Discovery DXA, clinicians see that the fracture shown in vertebral assessment (above left) puts women at increased risk of fracture again; this scan (above right) shows severe abdominal aortic calcification. |
Hologic Inc (Bedford, Mass) has received FDA clearance to use the visualizations attained by its Discovery Dual Energy X-ray Absorptiometry (DXA) system as indicators of abdominal aortic calcification (AAC), which has been proven in clinical trials to be a predictor of stroke and coronary heart disease, as well as other types of cardiovascular disease.
The Discovery system currently is used for fast, precise analysis of the major diagnostic indicators of osteoporosis: bone mineral density and vertebral fractures. Now, physicians are approved to use the same low-dose, 10-second instant vertebral assessment (IVA) test to look for AAC.

|
| The Discovery DXA performs a fast scan for osteoporosis; it now can be used to look for an indicator of heart disease as well. |
AAC refers to arterial calcification of the abdomen in particular. The presence of calcium buildup in the arteries can cause heart and circulation problems. And with heart disease and stroke as the leading causes of mortality in older men and women, early diagnosis—like that enabled by Discovery’s new AAC screening capability—becomes crucial.
Clinical studies show that AAC represents a risk factor for postmenopausal women in particular. As that same demographic group is also at the greatest risk for osteoporosis—and, therefore, is the population most often subject to the IVA test—being able to use the same image for two types of diagnostic screenings represents an unusual coincidence for health care professionals.
—C. Vasko
Partnership Aims to Increase Detection Rate of Lung Lesions
Philips Medical Systems (Andover, Mass) has entered into a strategic partnership with EDDA Technology Inc (Princeton Junction, NJ) to provide advanced DR solutions to assist in the detection of lung lesions. According to the terms of the agreement, Philips Medical has licensed EDDA’s IQQA-Chest, which is designed to help clinicians identify, quantify, evaluate, and report pulmonary nodules.

|
By bridging the gap between the clinician’s interpretation based upon patient-specific knowledge and computer analysis of the information captured by the x-ray, IQQA-Chest helps clinicians increase their performance while improving the efficiency for the analysis of each image. In clinical environments, the solution has been shown to increase the discovery rates of small nodules up to 85% in comparison to detection without IQQA assistance, which varies between 35% and 65%.
IQQA-Chest will be available in combination with the entire Philips Medical DR portfolio, including DigitalDiagnost, the company’s direct DR solution. The product initially will be available in the United States and China, as it has received regulatory clearance in both countries, and will become available in additional countries starting in 2007.
“Through our partnership with EDDA Technology, we’re providing clinicians with a new set of tools to aid in their detection of lung lesions at an early stage,” said Peter Reimer, global marketing director of general x-ray for Philips Medical.
Running the Numbers
$678 million is the earned revenue of the combined DR and CR market in the United States in 2005, according to the “US Digital and Computed Radiography Markets” report from Frost & Sullivan (Palo Alto, Calif). The report projects that the market will reach $977 million in sales in 2012. Visit www.medicalimaging.frost.com for more information.




