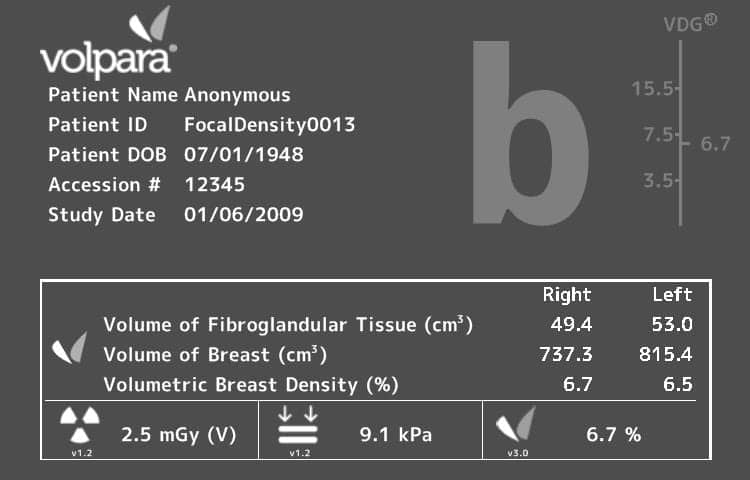
Originally cleared for use by the FDA in 2010, Volpara Solutions technology is currently in use in 31 countries, which have used the software to analyze breast density in more than 7 million women.
The new clearance covers VolparaDensity version 3.1, which has been specifically designed to correlate to the new Fifth Edition of the Breast Imaging-Reporting and Data System (BI-RADS) Atlas recently issued by the American College of Radiology. The 5th Edition Atlas was updated to require “an overall assessment of the volume of attenuating tissues in the breast, to help indicate the relative possibility that a lesion could be obscured … and that the sensitivity of examination thereby may be compromised by dense breast tissue.” Development of Volpara’s new software included a reader study which was used to optimize VolparaDensity performance to the BI-RADS 5th Edition.
Volpara will showcase VolparaDensity 3.1 software and other new advances to its suite of quantitative breast imaging tools at the upcoming Radiology Society of North America meeting, in booth 2377 of the South Hall.
“This updated FDA clearance is significant as it supports the ability of Volpara to analyze breast density from both digital mammography and tomosynthesis images using the BI-RADS 4th Edition scale that radiologists are familiar with and also the new BI-RADS 5th Edition that a growing number of radiologists are migrating to,” said Ralph Highnam, CEO, Volpara Solutions. “This is very exciting as it also comes right on the heels of an independent study recently published by researchers from the Netherlands that demonstrated that Volpara Density Grades showed substantial agreement with visual BI-RADS 5th Edition scores.”
For more information, visit Volpara Solutions.