Through an exclusive distribution agreement made in January 2015, Konica Minolta is handling commercialization of the product in Japan, which has the third largest ultrasound market in the world. According to the company, Konica Minolta demonstrated record sales last quarter, and with the regulatory approval, Konica Minolta looks to take an even greater share of the Japanese ultrasound market.
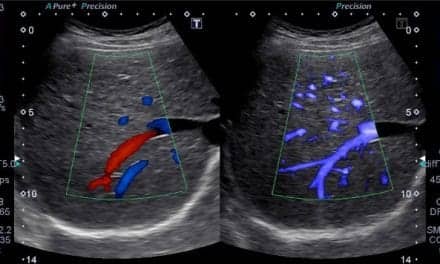
The Aixplorer provides real-time ShearWave Elastography to quantify tissue stiffness in a broad range of clinical applications, such as breast, liver, prostate, thyroid, and musculoskeletal assessments. Physicians use tissue stiffness to help identify potentially malignant or other diseased tissue. In addition to functionality for quantifying tissue stiffness, the Aixplorer also provides UltraFast Doppler that combines color flow imaging and pulsed wave Doppler into one exam, providing physicians with exam results simultaneously and helping to increase patient throughput.
“We are thrilled to have accomplished this regulatory approval, an important milestone that is a reflection of Konica Minolta’s and SuperSonic Imagine’s strong partnership,” said Jacques Souquet, founder and chief innovation officer of SuperSonic Imagine. “This technology is positioned to revolutionize ultrasound imaging in Japan by providing physicians with detailed, real time information in a broad range of clinical applications.”
For more information, visit SuperSonic Imagine.
Get AXIS e-newsletters free. Subscribe here.