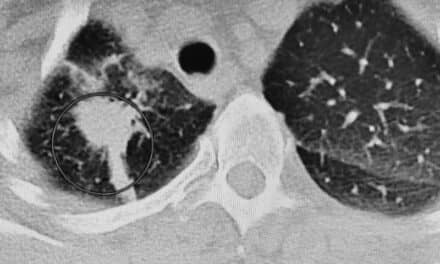
Future of PET Imaging Is Linked to Drug Development
Optimizing the PACS Investment
Adapting to New Technology
Extracolonic Findings Add Cost to Virtual Colonoscopy
Saving Money with CE-MRA: Study Finds DSA Considerably More Expensive
Future of PET Imaging Is Linked to Drug Development

|
| “One of the hopes is that as we get more of these molecularly targeted therapies, PET would give us a means to indicate early on whether we’re targeting what we think we’re targeting and whether the therapy is working or not.” —Simon R. Cherry, PhD University of California, Davis |
At the 2006 Annual Meeting of the Society of Nuclear Medicine (SNM), held last June in San Diego, Simon R. Cherry, PhD, professor of biomedical engineering at the University of California, Davis, presented his technological predictions about the future of PET imaging. His ideas are reiterated in an article in the November issue of the Journal of Nuclear Medicine.1 Axis Imaging News spoke with Cherry about the growing importance of the modality.
IE: PET imaging has become a key modality in oncologic imaging. Where does its future diagnostic potential lie?
Cherry: One of the hopes is that as we get more of these molecularly targeted therapies, PET would give us a means to indicate early on whether we’re targeting what we think we’re targeting and whether the therapy is working or not. One of the reasons gene therapy has run into so many problems is that once you put a therapeutic gene into a patient, it has not been easy to monitor what’s going on. If we had the ability to show which of the cells are expressing the gene at what level, and we were able to monitor that over time, that would be a very powerful help.
IE: What are your expectations for PET/MRI?
Cherry: I see it being a very powerful combination. Clinically, the big challenge is that it’s going to cost a lot of money. The question is: can we demonstrate that the diagnostic benefits outweigh the additional cost of combining these two instruments together? I think the jury’s going to be out on that for a while.
IE: Why is there not a software fix for fusing PET images and those from other modalities?
Cherry: Obviously, we have lots of PET scanners out there, and lots of MRI scanners. So, why not take the patients to the first machine, do one scan, then take them to the second machine and do the other scan, and then just use these computer algorithms to overlay the two images? For the brain, that works pretty well, because the brain is held in place by the skull. But for the rest of the body, that’s absolutely not true. If you lie down on two different couches, your liver will be in quite a different position. And then things change over time. The bladder fills up, changes shape, and moves the organs around it. So, it actually becomes very difficult to localize things using that approach. I think the success of PET/CT speaks to that; it tells you that PET/CT is doing something that just couldn’t be done with the two modalities separately.
IE: What are your thoughts on today’s PET software, and how do you see it advancing?
Cherry: I think it’s pretty good. The way we use PET in the clinic today is fairly unsophisticated in some senses. We inject FDG, we wait a little while, and then we take a single static snapshot of where the FDG is in the patient. Many of us in the research area would hope that maybe at some point in the future, we’ll take more advantage of the dynamic nature of the data and use temporal information in diagnostics as well. On the other hand, when you’re doing clinical tests, you want something that’s simple. So, in the software, I think there’s somewhat of a divergence between the need for simple clinical diagnostic tests versus the power of the imaging technologies.
IE: If computer processing speed continues to increase, will simplicity no longer be an issue?
Cherry: Oh, it definitely is [going to increase], but it’s the human side of it that’s actually the determining factor. It’s one thing to present a physician with a set of slides to look at, a volumetric data set. Now, if you add the time demand, they have to look at multiple slices plus the function of time. How do you distill that down into something that can easily detect disease?
IE: In 10 years, when a patient goes in for a PET scan, what will her radiologist be able to tell her?
Cherry: I think there are going to be more early diagnostic markers coming from simple blood tests and urine tests, because those are the only technologies we can do on the mass population. I see imaging as the secondary screen. We’ll say, ‘It’s likely that there’s a problem, and now we need to characterize that problem.’ A lot of it depends on where the therapies go, too. If we really head toward this vision of individualized medicine, then things like PET become very important, because we need to know the molecular basis of the disease in that patient. Once we really have an array of drugs and they’re molecularly targeted, we’re going to need to know the molecular basis of disease to know which drugs to prescribe for the patient, and I think PET will play an expanded role in diagnosis. Our future, I think, is very much tied to the future of drug development and what happens there.
Cat Vasko is associate editor of Axis Imaging News. For more information, contact .
Reference
- 1. Cherry SR. The 2006 Henry N. Wagner lecture: of mice and men (and positrons)—advances in PET imaging technology. J Nuc Med. 2006;47:1735–1745. Available at: jnm.snmjournals.org/cgi/content/abstract/47/11/1735. Accessed December 7, 2006.
Optimizing the PACS Investment
Erlanger Health System applies lean-thinking methodology
By Dana Hinesly

|
| “[This process has helped] us evaluate whether we are properly managing our resources, so we can continue to be competitive in the marketplace and viable to our institution.” —Byron Stutz Erlanger Health System |
It is no longer a choice but a necessity: If you perform medical imaging, you need PACS. But not all PACS implementations are successful, and no systems are plug-and-play. The amount of coordination and planning that happens before implementation goes a long way to determining the level of success that the technology brings to an organization.
“Industry consultants report more than half of facilities that installed PACS over the past 3 years have not realized projected productivity and revenue gains,” says Diana Nole, general manager of business strategy, product management, and marketing for Kodak’s Health Group, Rochester, NY.
Earlier this year, the Erlanger Health System, Chattanooga, Tenn, enlisted the services of Kodak Business Diagnostics Services (KBDS) to evaluate its current workflow and eliminate superfluous steps in preparation for the organization’s migration to digital imaging, including a new Kodak Carestream PACS. The conversion is the result of a multiyear, multimillion-dollar commitment to move all imaging modalities to DICOM.
“Once funding was approved, our chief information officer began a search for a solution we could afford, but also one we could migrate to in such a way that we could decrease some of our operational costs,” says Byron Stutz, chief technologist of Erlanger’s radiology services. “We were specifically seeking a PACS vendor that could provide a vehicle to remove unnecessary work in the department and streamline our workflow, making it as radiologist-friendly as possible.”
As part of the process, representatives from Kodak conducted multiple site visits, evaluating procedures from both an overall and a fine-detail level. The resulting changes cover just as wide a gamut—ranging from the elimination of single forms to determining the causes of registration bottlenecks.
“Lean-thinking methodology involves an established system of measurements and a process that produces precise estimates and results,” says David Spencer, worldwide manager of healthcare business solutions at Kodak’s Health Group. “Our service looks at the impact of a new imaging workflow on all users, including those inside and outside of the department.”
All of the analyses and changes are geared toward maximizing the benefits of PACS from the start, and they are flexible enough to accommodate specific demands of individual facilities. One example is Erlanger’s pediatric component.
“We think we may not meet Kodak’s benchmarking level for staffing in pediatrics, because we usually have two technologists available to perform the examination and help both the child and parents through the procedure. KBDS understands that pediatrics has a different requirement for technical staffing,” Stutz says.
The consultants were able to identify other hospitals that not only had the same technical staff but also had additional nontechnical staff after hours. “What we found was, compared to many other places, we were quite efficient,” Stutz notes. Providing such benchmarking information is one of the key components that KBDS brings to its clients. Based on a proprietary national database of more than 200 health care institutions, these benchmarks helped Erlanger accurately measure the facility’s performance differences and customer service in contrast to comparable facilities in the region.

|
| Erlanger is comprised of five campuses and conducts 300,000 imaging studies per year for more than 1,000 referring physicians. |
“We probably could have done some of this ourselves; however, we don’t have the time or the staffing, nor do we see the range of sites that Kodak sees. We didn’t want to just bring the system live and risk adding clutter and chaos to our environment,” Stutz notes. “Working with an objective third party helps us validate what we think we know. It also helps us evaluate whether we are properly managing our resources, so we can continue to be competitive in the marketplace and viable to our institution.”
Erlanger also is focused on becoming more customer friendly, remaining competitive by ensuring that not only are its radiologists eager to use its facilities, but that it also becomes a preferred treatment destination for patients.
Although much of the evaluation and suggested steps have been completed, the PACS has not been implemented as yet. Some suggestions prepared as part of KBDS for Erlanger include:
- Create an electronic version of the imaging study requisition form. In the PACS world, images will move faster than the requisition, so this form needs to be electronic in order to achieve the desired report turnaround time.
- Do not assign radiology reports to individual transcriptionists. Use a first-in, first-out process to streamline reporting.
- Stop printing radiology reports and putting them in patient jackets. They already are available online; if users need it, they can print it out.
- Contact patients one time for scheduling, insurance, preregistration, and screening questions. Making multiple contacts, which occurs with the current process, reduces staff productivity.
Erlanger currently projects the PACS will be online by the end of the first quarter of 2007. Also, Kodak is providing backup power supplies and disaster recovery planning.
Dana Hinesly is a contributing writer for Axis Imaging News. For more information, contact .
Adapting to New Technology
Build an optimum imaging solution by analyzing operational and facility requirements
By Bill Nation

|
New, more sophisticated diagnostic imaging equipment demands changes in operational requirements. As a result, facility requirements also must change. Hospitals partnered with design teams can analyze many variables to achieve an optimum imaging solution, but the hospital’s strategic operations plan should drive all facility planning. The strategic plan changes over time to meet the hospital’s mission and vision, so the facility plan should be revised accordingly. Planning for change itself is critical. For example, hospital rooms once served the same purpose over time. Today, hospitals must incorporate “flexible,” quickly convertible rooms that can serve different purposes.
Multiple factors define an imaging department’s best course. Each facility-unique factor provides critical information to evaluate current environment and operations, and to understand future requirements. Operational factors include patient demographics and acuity, patient programs or services, and staffing requirements. Facility factors include the number and variety of required imaging modalities, along with associated space requirements.
Operational Requirements
Such changes as an increasing number of elderly patients or modifications in other providers’ services could dramatically alter a region’s health care needs. Hospitals can use their existing detailed patient information to create maps of past patients’ points of origin. This information can define service areas by ZIP code or mileage within an established radius from the hospital.
When reviewed annually, the data can determine changes in patient volume due to shifting referrals, equipment or technology preferences, new providers, and appointment availability. This analysis should inform decisions to replace or add new technologies in the facility.

|
| At New York Presbyterian Hospital, it was important that the operation of the MRI would not disturb the functions of the surgical suite above or the pediatric cardiology suite below this space. |
Patient access to imaging services may come from multiple venues. Hospital-based programs provide services to inpatients, outpatient programs, and emergency departments. Although an imaging modality, such as CT, might serve each program adequately, each patient’s point of origin and physical condition require different service delivery requirements.
Thorough evaluation of physicians’ imaging preferences and requirements is critical to facility plan development. Physicians who specialize in certain procedure types, such as women’s or cardiac care, often have preferences regarding a facility’s location and type. They also might have additional support needs with associated space requirements.
Additionally, patients’ medical acuity impacts their ability to “wait,” as well as the type of space and staff required to support the waiting time. Inpatients typically arrive in the imaging area prepared and ready to receive service, but transport staff and private holding areas must support the imaging rooms. Outpatient access requires not only private holding areas, but also preparation or changing areas.
The shortage of clinical staff, which continues to impact hospital operations throughout the country, extends to radiologic technologists. To offset this problem, imaging equipment must be placed strategically to maximize both staffing and patient access efficiency. Such efficient operations contribute to a more favorable working environment, a critical factor for staff recruitment and retention.
Facility Requirements
Retrofitting a hospital to accommodate new, larger imaging equipment often is a challenge due to infrastructure age and spatial limitations. Many 1950s-, 1960s-, and 1970s-era hospitals present ceiling height limitations, as well as structural design that might not accommodate modern imaging units’ height and weight requirements.
Outpatient and ambulatory facilities built in the past 20 years offer more adaptability. These facilities allow easy patient parking, entry, diagnostics, and exit. Hospitals hoping to compete for these patients must offer similar accessibility. A plan for such a major change will require integrating all imaging needs and their expected changes.
Every piece of imaging equipment impacts space requirements, staffing, and patient services. Thus, all equipment should be inventoried. Specific records should include use, age, location, and portability, along with whether the unit is out of service or needing repair. The list can then be updated as equipment changes. The information provides a view of imaging as a complete entity.
The inventory also helps guide current and future imaging decisions. To this end, continual observation and analysis are imperative if imaging departments are to stay abreast of technology shifts. Changes in population and types of procedures, for example, may require higher-resolution equipment; new CT technology becomes available every 2 to 4 years.
Imaging equipment has a significant impact on space. Efficient imaging services depend largely on patient traffic management—specifically, separate inpatient and outpatient management strategies.
Outpatient volumes are greater than inpatient volumes. Separating the two populations may require one or more separate waiting and dressing rooms. This can enhance privacy, head off traffic conflicts, and minimize distances to the equipment. All these factors speed the diagnostic process.
Simultaneous Evaluations
To achieve the best planning solution, facility and operations plans should be coordinated simultaneously. Otherwise, missed opportunities, construction change orders, delays in project completion, and increased overall costs become unavoidable consequences.
Bill Nation is associate principal at Perkins and Will, Atlanta. He has more than 19 years of experience in helping clients find the optimum imaging solution for both new and renovation projects. For more information, contact .
Extracolonic Findings Add Cost to Virtual Colonoscopy
Researchers at Wake Forest University Baptist Medical Center, Winston-Salem, NC, have found that virtual colonoscopy has the potential to become much more costly than traditional optical colonoscopy because of its ability to detect abnormalities outside of the colon, which can create the need for additional testing. Richard S. Bloomfeld, MS, MD, assistant professor of internal medicine (gastroenterology) at Wake Forest, says his team of researchers found the added cost to be $231 on average.
“That’s maybe a 30% or 50% increase over the cost of the procedure,” Bloomfeld notes. “You need to consider that there are some good aspects to extracolonic findings and some bad aspects.”
Among the good aspects, of course, are early detection of such problems as renal cell carcinomas or abdominal aortic aneurysms, both of which can then be treated before they become life-threatening. On the other hand, Bloomfeld says, “If you find nodules or scarring that leads to more tests, which causes patients concern and costs a lot more money without leading to significant findings, that’s not so good. We’re trying to define the cost issue related to extracolonic findings. Other issues involve the psychological impact on patients—being told that they have these abnormalities—and that’s harder to quantify.”

|
Although many proponents of the procedure have been quick to hail CT colonoscopy as more patient-friendly than optical colonoscopy, Bloomfeld appends a note of skepticism to that potential advantage. “A virtual colonoscopy sounds great,” he agrees. “It sounds high-tech and noninvasive. But you still need to do a bowel prep, just like a conventional colonoscopy; and during a virtual colonoscopy, you put a tube in and pump the colon full of air. A standard colonoscopy also pumps air, but we give patients intravenous sedation medicine, so they usually don’t remember it. People often come in with the attitude that virtual colonoscopy is better, and then when they’ve had both, they often say that [optical] colonoscopy was more comfortable.”
Clinically, Bloomfeld favors tradition as well. “Right now, in my opinion, [optical] colonoscopy is a better test,” he says. “It is able to detect more polyps and remove the polyps in one setting.” But he is quick to note that virtual colonoscopy is going to have a role in the future of colorectal cancer screening—especially if it can be made more comfortable for the patient. “In 10 years, I think virtual colonoscopy will assume a bigger role in screening, when technology develops so it can be done without a bowel prep, which is not a pleasant thing,” he says. “When they develop technology such that virtual colonoscopy can be done without a bowel prep, that will encourage people to participate, and hopefully, we’ll have more people getting screened for colorectal cancer.”
The full results of Bloomfeld’s study were presented at the annual scientific meeting of the American College of Gastroenterology, held October 20–25 in Las Vegas.
—C. Vasko
Saving Money with CE-MRA: Study Finds DSA Considerably More Expensive
According to the findings of a study led by Joel W. Hay, PhD, associate professor of pharmaceutical economics and policy at the University of Southern California (USC), Los Angeles, significant cost savings can be achieved by using contrast-enhanced MR angiography (CE-MRA) instead of digital subtraction angiography (DSA) to image peripheral vascular disease for patients not requiring follow-up procedures within 30 days. The researchers also found that the Veterans Affairs (VA) system, whose records provided the data analyzed in the study, could have saved $13 million over 6 years by using CE-MRA.
“It was kind of surprising to us that there were so many patients [undergoing DSA] who had no subsequent procedures,” Hay says. “When we started the study, our assumption was that it would make sense to use DSA for a lot of patients because it would be immediately followed up with some type of additional intervention—some type of stent or surgical intervention. What we found was that even with the invasive DSA, very few people were getting the additional procedures within 30 or 90 days.”
The study, which was presented at the 9th Annual European Congress of the International Society of Pharmoeconomics and Outcomes Research, held in Copenhagen last October, reviewed VA data from 1999 to 2004 and found that treatment costs for patients undergoing CE-MRA were $3,500 to $4,300 lower over a 30-day period than treatment costs for patients undergoing DSA. Furthermore, during the 30 days following the procedure, 92% of the CE-MRA patients had no additional interventions or events, compared with 82% of the DSA group; 90 days out, that number dropped to 76% of CE-MRA patients and 65% of DSA patients.
“It really raised the question, ‘Why use this invasive and expensive imaging procedure if you’re not going to be doing anything anyway?’ ” Hay says. “If most of these patients had no additional procedures within 30 days, why use something that’s invasive and expensive? You’d be better off using something that’s much safer, less invasive, and cheaper.”
The USC researchers chose the VA’s pool of data because the records were more complete than those of any other health care system, but Hay believes the results represent a fair cross-section of what goes on in most hospitals. “In talking to the radiologists in our group, they would say that this is not that atypical. One of the reasons for that is that many of these VA hospitals are teaching hospitals for a lot of academic medical centers, so the practices there are not that different from the practices elsewhere.”
Hay cites slow-to-change radiologists as the most likely culprit behind the inefficient practice. “Physicians tend to be somewhat conservative, and they don’t adapt to new technology as quickly as they should,” he says. “What we’re hoping is that as more publicity gets focused on this issue, the VA and other systems will start to look at it more carefully.”
—C. Vasko