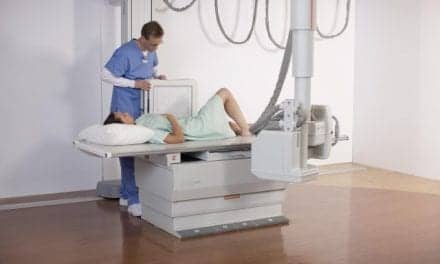
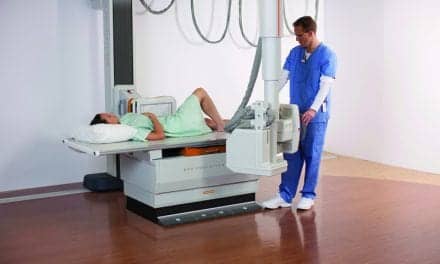
Rayence Inc. has announced US Food and Drug Administration clearance of its the digital universal radiography system. Sporting a unique compact design, the RU-3000 is the first U-arm system to be marketed by the company in the United States.
Rayence Inc. has announced US Food and Drug Administration clearance of its the digital universal radiography system. Sporting a unique compact design, the RU-3000 is the first U-arm system to be marketed by the company in the United States.
The system, whose small footprint allows for easy installation, boasts a dual telescoping arm movement that permits installation in settings having ceiling heights of just eight feet.
In addition, the RU-3000 has fully motorized movements for SID, arm rotation, height, and detector angle, which can be automatically programmed to user-specific radiographic positions through an intuitive touchscreen or by remote control.
The system comes with a 17 inch by 17 inch cesium detector, along with Rayence’s XmaruView workstation. Well-suited for all imaging environments, especially orthopedics, imaging centers, and urgent care, the RU-3000 features a durable Bucky design with an easily removable grid, patient safety anti-collision sensors, and a mobile table.
“The FDA’s clearance of RU-3000 is a major step in our effort to broaden Rayence’s presence within growing market segments,” said Bill Cioffi, vice president of Business Development at Rayence. “It is an essential first step in us achieving our future business goals.”
Get AXIS e-newsletters free. Subscribe here.