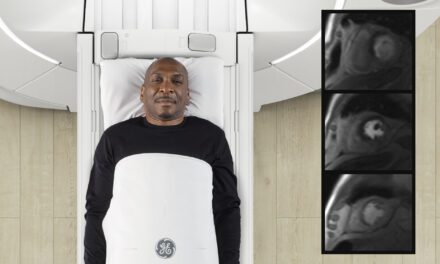
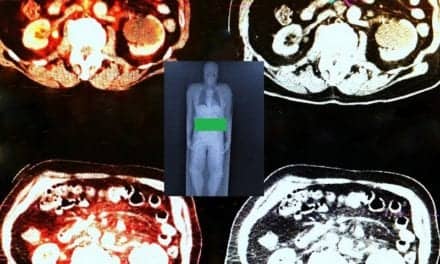
A pair of articles published in the May issue of The Journal of Nuclear Medicine illustrate the promise of the novel FAPI radiotracer in diagnosing, staging, and treating multiple types of cancer. Results from the largest study of patients undergoing 68Ga-FAPI PET demonstrate the superiority of 68Ga-FAPI over standard 18F-FDG PET in evaluating multiple types of cancer.
In a separate study, a newly designed FAPI-targeted treatment was found to suppress tumor growth in several common cancers in a preclinical setting. These advances have strong potential to provide more precise staging and management for cancer patients.
Cancer-associated fibroblasts are involved with tumor growth, migration, and progression. A sub-population of cancer-associated fibroblasts express fibroblast activation protein (FAP). FAP is prominently expressed in solid tumors but virtually absent from healthy tissues, which makes it an attractive diagnostic and therapeutic target for fibroblast activation protein inhibitors (FAPI).
In the first study, 324 patients with 21 different tumor types underwent 68Ga-FAPI PET over a three-year period; 237 of them also received 18F-FDG PET imaging. Researchers compared 68Ga-FAPI PET and 18F-FDG PET in terms of uptake across tumor entities. They also examined whether there was a correlation between 68Ga-FAPI uptake on PET scan and FAP expression on stained tissue samples.
Uptake was significantly higher for 68Ga-FAPI as compared to 18F-FDG in primary lesions of pancreatic cancers and sarcoma and in metastatic lesions of pancreatic cancers. 68Ga-FAPI PET showed superior detection for local and regional disease in sarcoma as well as for distant metastatic disease in sarcoma and cancers of the pancreas, head and neck, bile ducts, lung, and bladder. A positive correlation was also found between radiotracer uptake and FAP expression levels in the tissue samples.
“68Ga-FAPI PET can be used as a tool for diagnosis of tumors, with the potential for more precise staging and management of patients with the aforementioned tumor entities,” says Nader Hirmas, MD, ScD, a PhD candidate at the Department of Nuclear Medicine at Essen University Hospital in Germany. “It could also be used as a tool to screen patients who would potentially benefit from FAP-directed radioligand therapy.”
In the second study, researchers developed a new FAP radiopharmaceutical therapy that targets naturally occurring cancer-associated fibroblasts. “In previous tumor models, researchers altered cancer cells to express high levels of FAP as a target for therapy. In this study we sought to create a treatment that would be effective in the natural tumor environment,” says Philip S. Low, PhD, presidential scholar for drug discovery and Ralph C. Corley distinguished professor in the Department of Chemistry at Purdue University in West Lafayette, Indiana.
Researchers used a modern bioanalytical method (single cell RNA-seq) to determine which cells in 34 human breast, ovarian, colorectal and lung tumors expressed FAP. Two radiopharmaceutical conjugates—FAP6-DOTA and FAP6-IP-DOTA (the latter of which contains an albumin-binder to prolong circulation and improve tumor uptake)—were developed and tested on human FAP-expressing cells. Radiopharmaceutical therapies of 177Lu-FAP6-DOTA and 177Lu-FAP6-IP-DOTA were also investigated in a mouse model.
FAP was found to be overexpressed on 5% of human tumor cells; cancer-associated fibroblasts constituted 77% of this FAP-subpopulation, while two percent were cancer cells. FAP6-IP-DOTA was shown to exhibit high FAP affinity, prolonged circulation, increased tumor uptake, and minimal retention in healthy tissue. In addition, single doses of 177Lu-FAP6-IP-DOTA suppressed tumor growth by nearly 50% in all tested tumor models without causing reproducible toxicities.
“These data suggest that this newly designed FAP-targeted radiotherapy should be capable of treating many more types of human cancers in which the FAP expression is limited to only the cancer-associated fibroblasts,” notes Spencer D. Lindeman, PhD, visiting scholar in the Department of Chemistry at Purdue University. “This could be a powerful and versatile tool for the field of clinical nuclear medicine.”