Mention virtual colonography (VC), and two studies come to mind: that led by Perry J. Pickhardt of the University of Wisconsin (Madison) and published in 2003, which found VC comparable to conventional colonoscopy and even better able to locate polyps greater than 8 mm in diameter,1 and that led by Peter B. Cotton from the Medical University of South Carolina (Charleston), whose results published in 2004 found VC’s sensitivity for various-sized polyps to be significantly lower than conventional exams.2
Mention virtual colonography (VC), and two studies come to mind: that led by Perry J. Pickhardt of the University of Wisconsin (Madison) and published in 2003, which found VC comparable to conventional colonoscopy and even better able to locate polyps greater than 8 mm in diameter,1 and that led by Peter B. Cotton from the Medical University of South Carolina (Charleston), whose results published in 2004 found VC’s sensitivity for various-sized polyps to be significantly lower than conventional exams.2
|
One year later, the debate over the findings still rages. As a result, there is, generally, no reimbursement and low adoption. But while more extensive data is compiled that compares VC to conventional colonoscopy, it’s fair to wonder why VC can’t at least have spot number two. “VC is better than the barium enema and should be advocated as the next best thing,” says Joseph T. Ferrucci, MD, professor and chairman emeritus of radiology at Boston University School of Medicine.
Images taken of the colon with the latest CT technology are crisper, showing more than images that were taken with earlier equipment. Now, with 64-slice scanner and 3-D imaging technology, it’s easier to spot potentially cancerous polyps and lesions. Future technology and software improvements promise even greater clarity and quicker results.
With the development of prepless exams, VC?already seen as more comfortable and convenient?will make regular screening far more palatable for many patients. Low screening compliance is still a major factor in colon cancer-related mortality that, according to many, could be minimized and quite possibly eliminated with regular colon screenings. Although researchers and clinicians seek data and reimbursement, word about the procedure will need to be spread; however, experts don’t necessarily agree on who best to promote it.
Selling It
|
Ferrucci believes that radiologists are best positioned to publicize information about VC. “If you take mammography as an example, radiologists have educated the public,” says Ferrucci, who notes that efforts to work with agencies and associations are under way. “Ultimately, it will have to be a combination of colon cancer patient advocacy groups, associations like the American Cancer Society [ACS of Atlanta], the media, and individual physicians and researchers.”
According to Matthew Barish, MD, director of the 3-D and Image Processing Center in the Department of Radiology at Brigham and Women’s Hospital, Harvard Medical School (Boston), a statement by an association?such as the American College of Radiology (ACR of Reston, Va)?would help spread the word. “Guidelines that cover cancer could be updated. VC can replace or be listed side by side with barium enemas. These societies have a lot of weight,” Barish explains.
But it’s possible that primary care physicians (PCPs) have even more. Patients typically see this physician first. “They are responsible for assuring patient cancer screening. They are the best individuals to publicize VC to the patient,” Barish says.
Unfortunately, PCPs might be unaware of the procedure or are hesitant to suggest its use since it is neither endorsed nor reimbursed, according to Paul Schroy, MD, MPH, professor of medicine at Boston University School of Medicine and director of clinical research?GI at the Boston Medical Center.
Adds Ferrucci, “PCPs are confused by the literature put out by the American Gastroenterological Association [AGA of Bethesda, Md] and other GI associations that has attacked VC without providing other choices. The studies are confusing.”
To get the medical community on board, experts agree that more articles need to be published, and more presentations need to be developed. “There needs to be a lot of supportive literature showing the benefits,” says Milind D. Dhamankar, MD, manager of clinical research collaborations at Siemens Medical Solutions (Malvern, Pa).
And, there will need to be reimbursement. CT is expensive, and consumers might not be willing to pay for it. In some areas, VC is reimbursed when used for diagnostic purposes, but not yet for screening.
“VC needs to be reimbursed for screening,” says Loke-Gie Haw, clinical marketing manager of the CT division at Siemens Medical. “Most imaging centers will introduce it. As we stand now, they already are doing so but are only getting reimbursement for clinical diagnosis?not for screening. Once it is reimbursed, we’ll need more multicenter trials to prove its efficacy.”
 |
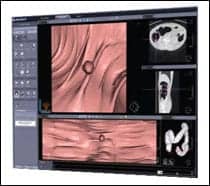
| The banana segment (left) is analogous to several sophisticated colon straightening/splitting projects that mathematically straighten colon segments, then cut each segment into halves at arbitrary angles, resulting in four separate edges. What straightened filleted topographic overview (SFTO) does is fundamentally different. SFTO (center) skins the banana, then looks at?as well as through/beyond?the mucosal surface with a unique subsurface view (SSV). These SFTO/SSV images (right) from a sigmoid segment show an over-sampling correction for edge distortion regarding potentially missable polyps on the two edges (not four) of SFTO-flattened images. |
Proving It
Some studies to prove efficacy are in progress already. One such study is that by the ACR Imaging Network (ACRIN of Philadelphia), which seeks to evaluate the accuracy of VC in a multicenter setting.
“A couple of studies are under way, but the results won’t be available until later, possibly 2008 or 2009,” Ferrucci says, adding that he expects VC will be more accepted by that time. “Colonoscopists will have to be comfortable relating it to their practices. Some might even buy their own scanners.”
In the meantime, debate rages over the results of studies already published. “When VC is performed by people experienced with the technology and latest software, it compares favorably,” Schroy says. “But there have been widespread results suggesting a sensitivity issue.”
Barish adds, “In the Cotton study, the disappointing results can be explained by the poor design?older technology and untrained readers, but in studies with improved design, the variable results are harder to explain.”
A meta-analysis of 33 studies led by Brian P. Mulhall, MD, MPH, former chief of the liver clinic at the Walter Reed Army Medical Center (Washington), attempts to offer an explanation. “Previous reports have implied that the differences in test performance among studies of CT colonography are related to the CT colonography technology used, the type of contrast medium, the mode of imaging, and the expertise of the radiologists reading the images. The available data are sufficient only to suggest that multi-detector scanners, mode of imaging, and low collimation width affect test performance. Many other possible sources of false-negative results exist, including limitation in technology and technique, insufficient resolution, poor bowel distention, poor preparation, breath-hold artifacts, misinterpretation of stool or folds, sessile or flat polyps, paired lesions, software limitations, and errors in reading.”3
One problem with all of the studies is that by the time they are completed, new technology has been developed that helps to further improve images. For instance, by the time the current studies are completed, Ferrucci expects the exam to be prepless and reimbursed.
Improving It
|
“Many patients don’t want to take the horrible-tasting preparatory goop,” Ferrucci observes. Prepless VC eliminates the need for this laxative component. Instead of cleansing the colon of all stool, fecal matter is tagged with a contrast agent.
Tagging, in conjunction with software, can better differentiate lesions from stool. “More often than not?even with cleansing?some fecal matter will still be attached to the colon walls. In VC, it is difficult to differentiate true polyps. If the patient drinks a barium preparatory solution, the contrast agent sticks to the fecal material, appearing as a bright color on the scan. Using software, the colon can then be electronically cleansed of fecal matter,” says Haw, who reveals that Siemens Medical is researching contrast agents for this purpose.
Other expected advances use software as well. A major focus now is CAD. Barish predicts, “CAD will be the next thing and will be commercially available within the next year.”
Haw shares that most CAD products are still works in progress, but that Siemens Medical did release a first version last year, syngo Colonography with PEV (polyp-enhanced viewing). The product has been cleared by the FDA as a second reader. “The software is designed to help radiologists pick up suspicious areas,” he says.
Even as a second reader, CAD serves to improve not only accuracy but turnaround as well. Other works in progress promise even more efficiency.
William Glenn, MD (Manhattan Beach, Calif), is leading one of the teams working on flattening software, which opens the colon to permit a topographic overview. Glenn’s project opens the colon like, essentially, a Fruit Roll-Up. “Conventional flattening software halves the colon and opens it,” says Glenn, illustrating his point with a banana. “Traditionally, the banana is halved. With this new approach, the peel is halved and flattened.”
Polyps and lesions are easier to spot with this method, particularly when they are hidden behind a ridge or haustral fold. The software can zero in, magnify, and measure questionable areas for closer viewing. Improvements in CT technology have only made locating polyps easier, according to Glenn.
“The differences in images taken with a 64-slice CT scanner and an older version, even 16-slice, is remarkable,” he says. The clarity and flattening software combine to make it possible to rapidly scan the colon, completing a read in seconds. Identifiable shapes mean that the software will likely work very well with CAD systems, and a centrally linked network could allow expert readers to conduct many exams through teleradiology, Glenn says, envisioning an accurate, efficient, and profitable system that will benefit everyone.
 |
| Coronal reformatted images show significant visual differences from three distinct phases (A, B, and C) of recent CT advances. The “invisible” 32% of the colonic lumen not seen with fiber-optic colonoscopy (FOC) exams is noted by an asterisk (*), on the upstream or proximal side of haustral ridges, where the colonoscope is basically blind. This is schematically demonstrated in D, which shows two right colon simulated FOC view points, then counts visualized versus blind FOC pixels over a short forward distance. The average is 32% FOC blind spots. |
Using It
For now, only patients who have failed conventional colonoscopy are generally directed to VC. Other candidates include patients on blood thinners and children, according to Ferrucci.
Proponents would like to see VC obtain approval and reimbursement as a screening method; many believe VC endorsement by the medical community, coupled with the relative ease of the test, would encourage people to follow the ACS recommendations for colon screening. At the very least, proponents argue that VC is better than nothing.
The first stages of colon cancer exhibit few symptoms, and screening does save lives. The ACS notes4 that incidence rates of colon cancer decreased by 2.9% per year during 1998?2001?a decline attributed, at least in part, to increased screening and polyp removal. When detected at an early, localized stage, the 5-year survival rate of colorectal cancer is 90%. After it has spread regionally, the 5-year survival rate drops to 67%. If the cancer has metastasized further, the 5-year survival rate is a mere 10%.
Other statistics are just as grim. Colorectal cancer is the third most common cancer in men and women.4 The ACS estimates4 that 56,290 Americans will die of the disease this year and that 104,950 new cases will be diagnosed. Because the risk of developing colorectal cancer increases with advancing age (more than 90% of cases occur in those 50 years or older), the ACS recommends one of five screening programs for persons 50 years and older?see “The Guidelines,” below.
Yet research from the National Health Interview Survey (NHIS), administered by the Centers for Disease Control and Prevention (CDC of Atlanta), indicate that in 2000, fewer than half of US adults (42.5%) age 50 years or older had undergone a sigmoidoscopy or colonoscopy within the previous 10 years or had used a fecal occult blood test (FOBT) home test kit within the preceding year.
Calling It
Some suggest that politics, not polyps, are holding VC back. “VC should be added to the list of approved screening tests, but if it were to be added, it would compete with colonoscopy. Many hospital administrations and gastroenterologists are invested in colonoscopy, and it becomes a bit of a turf struggle,” Ferrucci explains.
But Schroy does not see VC ever replacing traditional colonoscopy. “VC is a good test, and I believe it will continue to get better. It’s a good option for those who avoid traditional colonoscopy and should be endorsed,” he says, while voicing concerns about prepless advances, facility capacity to support volume, and expertise. “Maybe not many centers or radiologists are performing it, so VC might play out like mammography with certified centers and be somewhat restricted.”
Barish adds, “After the Pickhardt study, I did think VC would be competitive, but then studies, such as Cotton’s, were published with disappointing results. Still, VC outperforms every other screening test except for traditional colonoscopy. Any patient who is willing and able to undergo conventional colonoscopy should be encouraged to do so. But if unwilling or unable, they should have the virtual exam. It’s the next best thing.”
References
- Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003; 349(23):2191?2200.
- Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multi-center comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004; 291(14):1713?1719.
- Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med. 2005; 142:635?650.
- American Cancer Society. Cancer facts and figures 2005. Available at: www.cancer.org. Accessed August 15, 2005.
The GuidelinesThe American Cancer Society offers screening guidelines for colon cancer. Beginning at age 50, both men and women at average risk for developing colorectal cancer should follow one of five testing schedules:
Source:American Cancer Society. ACS cancer detection guidelines. Available at: www.cancer.org. Accessed August 15, 2005. |
?WD |
Wren Davis is a contributing writer for Medical Imaging.