Imaging plays an important and growing role in managing the cancer patient at every stage of care: screening, diagnosis and staging, treatment, therapy monitoring, and lifelong follow-up. In fact, imaging has become a fundamental tool for cancer care. Traditionally used primarily as a critical diagnostic tool, imaging also is becoming increasingly intertwined with therapy—medical and surgical therapies as well as radiotherapy.

|
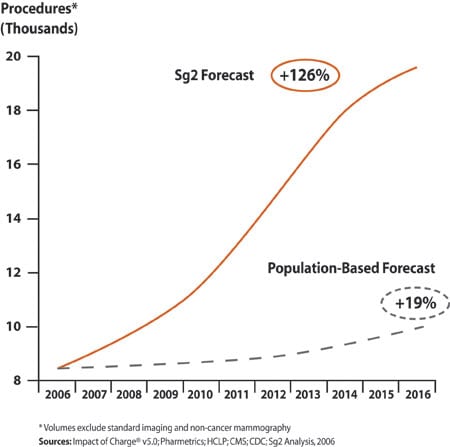
| Figure 1. National outpatient cancer imaging forecast for the US market from the Sg2 Imaging Forecast, US market, 2006–2016. (click image for larger view.) |
Indeed, the continued growth in cancer imaging will far outpace what population alone would predict (Figure 1). In 2006, advanced imaging techniques with CT, MR, PET, and SPECT represent 63% of imaging procedures for cancer patients. Due to the expanding utility of these applications, the procedure volume for these modalities will soar 189% by 2016, according to health care market forecaster and consulting firm Sg2, Skokie, Ill. The standard imaging methods, such as traditional x-ray and ultrasound, also will continue to grow at a rate slightly above population. According to Sg2, all imaging procedures for cancer will grow 126% in the next decade, compared to a population-based forecast of 19%.
For hospitals, this rapid growth has important implications. Imaging is often the first point of contact for the cancer patient with the institution. Cancer patients will receive multiple imaging studies during the course of their disease, mainly in an outpatient setting. Unlike freestanding imaging centers, hospital outpatient imaging programs can serve as a major gateway to the facilities’ other services.
Although a single screening mammogram may not be profitable, a positive diagnosis from it could lead to a patient who needs multiple imaging studies and therapies that would generate significant revenue over time for the hospital. A positive experience for a patient undergoing a screening study is an opportunity for the hospital to build its brand and establish that long-term relationship.
New Technologies Will Extend Screening
Mammography has shown that early detection of disease can improve the chances for successful treatment. In the next decade, imaging’s role in screening will evolve beyond mammography to include such applications as CT lung screening of high-risk patients, virtual colonoscopy, and even rapid whole-body MR studies.
Computer-aided detection (CAD) will be important as a process enhancer in all of these studies. This technology is still at an early stage of development and can be expected to evolve and improve significantly over the decade.
Screening programs always will be low-margin tests, so they must be conducted efficiently and at the lowest possible cost. Effective screening programs require attention to workflow and well-designed processes that optimize the patient experience and readily integrate the technology and staff performing the examination with the clinical need.
Since imaging examinations are generally high-cost tests, reimbursement will be a major driver of the growth trajectory of imaging screening studies. Therefore, protocols for appropriate patient selection and demonstration of improved clinical outcomes from screening will be important to secure reimbursement from payors.
Advances Will Improve Diagnosis and Staging
All modalities have a role to play in diagnosis and staging tumors, with CT, MR, and PET playing a dominant role virtually anywhere in the body. The transition of CT from a display of parallel axial slices to volumetric imaging (with 16-slice and larger scanners) is changing how oncologists rely on images in diagnosis. Now, 3D views with quantitative information on tumor volume and the relationship of the tumor to surrounding structures can be visualized easily.

|
| Figure 2. Imaging volume distribution in the US market in 2006. Projection based on Sg2 Imaging Forecast, US Market 2006–2016. (click image for larger view.) |
Fusion imaging techniques allow angiographic data to be mapped to these studies or permit the anatomic volume to include functional information on tumor metabolism from molecular imaging examinations. Experts are increasingly able to define a tumor type through functional imaging tests. The specificity of imaging examinations will improve dramatically as targeted contrast agents and new radiopharmaceuticals come to market and as more institutions become familiar with advanced MR studies, such as spectroscopy at 3T field strengths.
As the decade evolves, it will be easier to exploit the specific strengths of different modalities in the diagnostic workup. For example, breast cancer imaging will include such techniques as dynamic contrast MR mammography, scintimammography, spectroscopy, and optical methods to complement the x-ray mammogram with information specific to identifying the tumor type and quantifying its metabolism. X-ray mammography itself will see improvements in both sensitivity and specificity with dual-energy techniques and tomosynthesis.
For many referring physicians, FDG-PET (fluorine 18-labeled deoxyglucose) already has established itself as the gold standard for staging disease, as it is highly sensitive to identifying metastases throughout the body. Correct staging of disease is essential to planning appropriate therapy, and PET has been found to frequently upstage disease when compared to other imaging tests, thus changing the course of treatment. As the decade develops and more physicians become comfortable with the technology, this modality will become the accepted standard of care for staging many tumor types.
The developing field of molecular medicine will provide such tools as disease-targeted contrast agents and radiopharmaceuticals that will enhance the specificity of diagnostic imaging tests and personalize each examination. (See Chelator-Based Imaging Technology) As the technology develops, the traditional biopsy will increasingly be replaced by these highly specific noninvasive studies.
While offering a wealth of valuable new options, these agents also will pose challenges for hospitals. Hospitals must be prepared to handle a growing inventory of agents, many of which will require radiation hot lab facilities for their storage and preparation.
Imaging’s Importance to Treatment Will Only Increase
Perhaps the greatest revolution in the role of imaging in patient management in the coming decade involves therapy. Whether the treatment plan includes surgery, chemotherapy, or radiation, imaging is often integral to the procedure. In surgery, CT and MR data sets are used for planning the procedure and providing the landscape for surgical navigation systems to guide the physician’s hand. New OR suites include imaging equipment in their design. Ultrasound and fluoroscopic x-ray are available today in virtually every surgical suite.

|
| Figure 3. Outpatient cancer services distribution based on 109 million procedures in 2006. Projection based on Sg2 Imaging Forecast, US Market 2006–2016. (click image for larger view.) |
Moreover, the technology of cone-beam CT will transform surgical imaging by providing volumetric data in special procedures rooms and, in the near future, on portable C-arm systems that can be moved from one operating room (OR) to another. Special-purpose OR suites for neurosurgery with MR and other advanced imaging capabilities will become used more widely. Miniature gamma and beta probes will be used in the wound to pinpoint surgical margins and confirm that all of the disease has been excised.
Already, radiotherapy makes heavy use of CT in treatment planning and simulation. Increasingly, MR and PET images will be fused to the CT planning data to provide additional information on tumor metabolism and structure as well as to tighten target volumes by better identifying lesion margins. This approach will permit higher therapeutic doses and dose distributions that are contoured to match the tumor metabolism. In addition, new therapy accelerators incorporate imaging in both 2D and 3D to allow visualization of tumor size and position at each treatment session, permitting the patient position and treatment plan to be adjusted to spare healthy tissue and optimize therapeutic dose over the entire treatment regime. As experience with this technology grows, it is likely that the therapeutic dose delivered at each treatment fraction will be increased, and the number of fractions required to complete treatment reduced accordingly.

|
| Figure 4. Outpatient cancer services distribution forecast for the US market in 2016. Projection based on Sg2 Imaging Forecast, US Market 2006–2016. (click image for larger view.) |
Interventional radiology suites are becoming important therapeutic tools in oncology with chemotherapy agents, embolization, and even radioactive seeds being delivered via catheter directly to the tumor. This minimally invasive technology enables more aggressive treatment for patients who, a few years ago, would be eligible only for palliative care.
The marriage of imaging and therapy is seen in new generations of therapeutic radiopharmaceuticals that employ imaging systems to personalize dose. These drugs typically are based on a monoclonal antibody that targets the tumor.
The treatment regime consists of several steps, starting with the injection of an initial “cold” dose that is followed by a small “hot” dose of the agent tagged with a radioisotope. A gamma camera is used to detect and quantify the dose distribution, allowing a customized dose plan to be implemented for each patient. A therapeutic dose of the hot agent is then given, with the results followed on the gamma camera.
The first agents of this type received FDA approval in 2002 and 2003. At least 20 other targeted radiotherapeutic agents in development are expected to enter clinical practice in the next decade.
Monitor and Follow-up to Span a Lifetime
Imaging is playing a growing role in monitoring the success of therapy. Many cancers present the physician with a variety of treatment options, and it often is unclear which choice is best for an individual patient. Often, treatment regimes are lengthy and have significant side effects, so assessing the effectiveness of treatment as early as possible can make a real difference in managing a patient’s care.
By looking for changes in a tumor’s size and shape, new functional methods of anatomic imaging attempt to assess tumor response before there is a structural change to the tumor. For example, MR spectroscopy can see changes to tumor metabolism early during the course of radiation therapy to assess whether the tumor is responding or is radio-resistant. FDG-PET is extremely sensitive to small changes in tumor metabolism and is now approved for reimbursement for treatment-monitoring applications by CMS under the National Oncologic PET Registry program. This application of PET will see tremendous growth in the next decade.
For the growing number of cancer survivors who have finished treatment, imaging also plays a recurring role. They undergo regular imaging assessments designed to detect or rule out recurrence or new disease for the duration of their lives.
Seizing the Opportunity
A strong imaging program is essential to any oncology program, given its vital role at each stage of patient care. Moreover, imaging is a portal to the organization and an opportunity to grow the brand. However, careful planning is essential for managing the significant growth in demand that looms for imaging services in cancer, especially for the advanced modalities, in the next decade. Inevitably, the integration of imaging and therapy will present challenges to the established order. For example, there are already anecdotal accounts of images intended for therapy setup on a linear accelerator showing new disease occurring during the course of treatment.

|
| Figure 5. Figure depicts consensus adoption of emerging technologies, which become more complex and tumor-specific over time. (click image for larger view.) |
Hospitals will have to address tough questions: What level of training is required for the radiation therapists who are using these images every day, and what processes must be put in place to ensure that the information in these images is effectively interpreted? Who should be delivering the new therapeutic radiopharmaceuticals? Is it the responsibility of medical oncology, nuclear medicine, or radiation oncology, as all three disciplines must be involved in the therapeutic process? Is interventional oncology a subspecialty of interventional radiology, or will this become a new discipline aligned with a surgical or medical oncology group?
Using technology wisely requires an investment in physician and technical expertise, the development of effective work processes, and careful attention to ever-higher standards of quality to maximize its clinical value. With these keys in place, hospitals will be poised to take advantage of the coming boom in cancer imaging.
Christopher J. Farr is vice president of the Imaging Intelligence Program at Sg2, Skokie, Ill. For more information, contact or visit www.sg2.com.