Researchers have developed a new, interpretable artificial intelligence (AI) model to predict five-year breast cancer risk from mammograms, according to a new study published in Radiology, a journal of the Radiological Society of North America (RSNA).
One in eight women, or approximately 13% of the female population in the U.S., will develop invasive breast cancer in their lifetime and one in 39 women (3%) will die from the disease, according to the American Cancer Society. Breast cancer screening with mammography, for many women, is the best way to find breast cancer early when treatment is most effective. Having regularly scheduled mammograms can significantly lower the risk of dying from breast cancer. However, it remains unclear how to precisely predict which women will develop breast cancer through screening alone.
Mirai, a state-of-the-art, deep learning-based algorithm, has demonstrated proficiency as a tool to help predict breast cancer but, because little is known about its reasoning process, the algorithm has the potential for overreliance by radiologists and incorrect diagnoses.
“Mirai is a black box—a very large and complex neural network, similar in construction to ChatGPT—and no one knew how it made its decisions,” says the study’s lead author, Jon Donnelly, BS, a PhD student in the Department of Computer Science at Duke University in Durham, N.C. “We developed an interpretable AI method that allows us to predict breast cancer from mammograms one to five years in advance. AsymMirai is much simpler and much easier to understand than Mirai.”
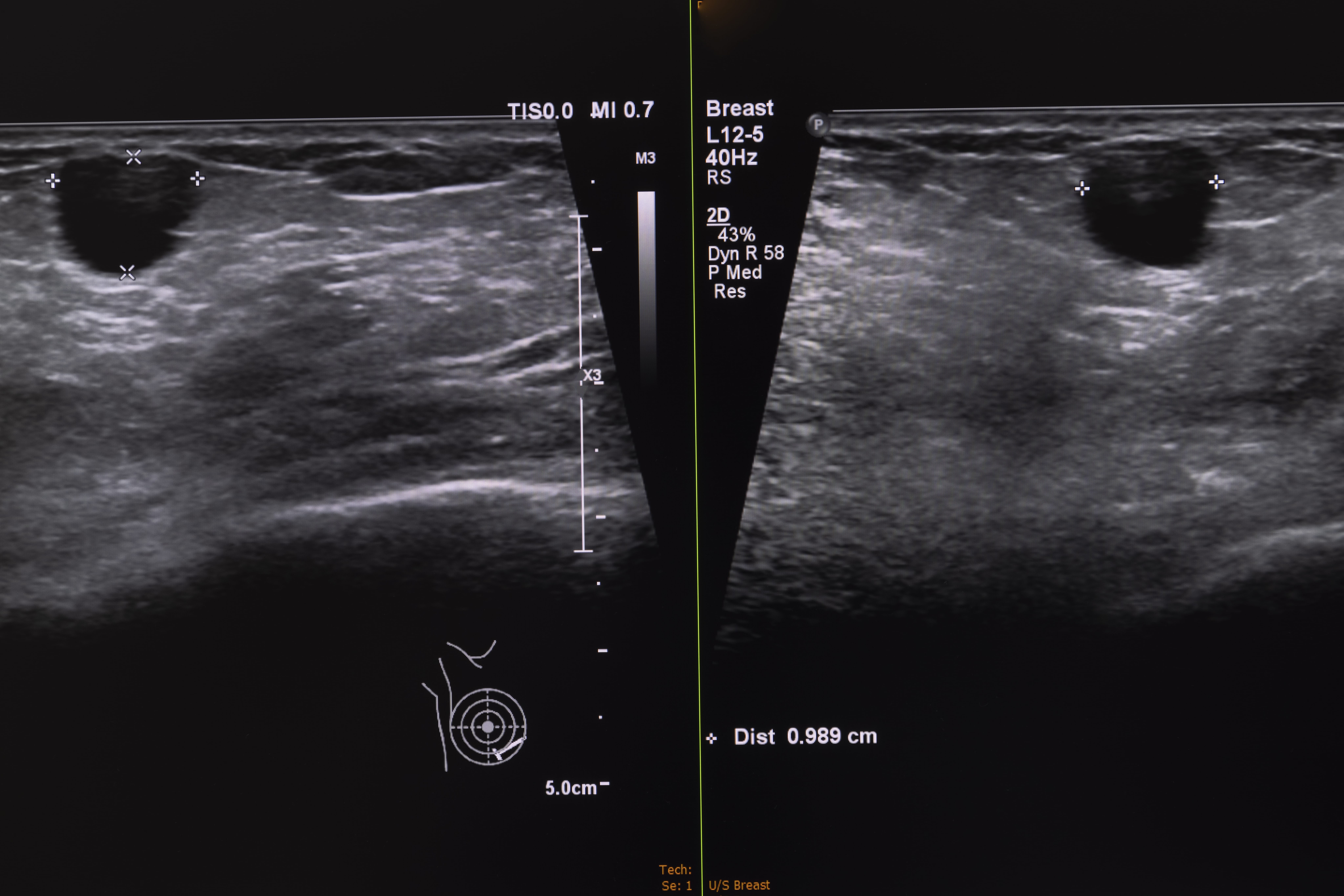
For the study, Donnelly and colleagues in the Department of Computer Science and Department of Radiology compared their newly developed mammography-based deep learning model called AsymMirai to Mirai’s one- to five-year breast cancer risk predictions. AsymMirai was built on the “front end” deep learning portion of Mirai, while replacing the rest of that complicated method with an interpretable module: local bilateral dissimilarity, which looks at tissue differences between the left and right breasts.
“Previously, differences between the left and right breast tissue were used only to help detect cancer, not to predict it in advance,” Donnelly says. “We discovered that Mirai uses comparisons between the left and right sides, which is how we were able to design a substantially simpler network that also performs comparisons between the sides.”
For the study, the researchers compared 210,067 mammograms from 81,824 patients in the EMory BrEast imaging Dataset (EMBED) from January 2013 to December 2020 using both Mirai and AsymMirai models. The researchers found that their simplified deep learning model performed almost as well as the state-of-the-art Mirai for 1- to 5-year breast cancer risk prediction.
The results also supported the clinical importance of breast asymmetry and, as a result, highlights the potential of bilateral dissimilarity as a future imaging marker for breast cancer risk. Since the reasoning behind AsymMirai’s predictions is easy to understand, it could be a valuable adjunct to human radiologists in breast cancer diagnoses and risk prediction, Donnelly says.
“We can, with surprisingly high accuracy, predict whether a woman will develop cancer in the next one to five years based solely on localized differences between her left and right breast tissue,” he adds. “This could have public impact because it could, in the not-too-distant future, affect how often women receive mammograms.”