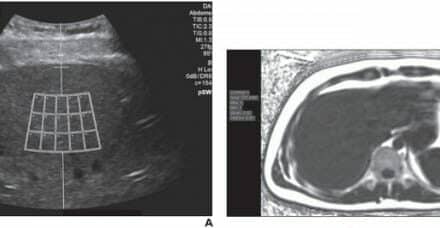
U-Systems, Sunnyvale, Calif, announced today that the somo•v® Automated Breast Ultrasound (ABUS) system has been approved by the US Food and Drug Administration (FDA) for breast cancer screening as an adjunct to mammography for asymptomatic women with dense breast tissue. With the approval, the somo•v ABUS system becomes the only device approved specifically for screening women with dense breasts.
Recently completed studies demonstrated that ABUS helps find about 30 percent more cancers in women who have normal mammogram, normal physical examination, and dense breasts. More than 40 percent of women have dense breasts. Dense breast tissue not only increases the risk of breast cancer up to 4-6 times but it also makes cancer more difficult to detect using mammography, according to multiple large studies. One study, published in the New England Journal of Medicine, showed 35 % of breast cancer goes undetected by mammography in women with dense breasts, as density masks the appearance of tumors. As breast density goes up, the accuracy of mammograms goes down.
Using proprietary technology to automate the ultrasound imaging process, the U-Systems’ somo•v ABUS system was developed specifically for the high-volume, breast cancer screening environment. The somo•VIEWer™ Advanced 3D Workstation enables fast, accurate review and archiving of patient exams, optimizing breast ultrasound screening workflow.