Recipient of the joint SNMMI Mars Shot and Lobular Breast Cancer Alliance fellowship will study an innovative approach that combines two non-invasive tests
The SNMMI Mars Shot Research Fund and the Lobular Breast Cancer Alliance (LBCA) announce that Marina Sharifi, MD, PhD, has been selected as the recipient of the joint SNMMI/LBCA Invasive Lobular Carcinoma Imaging Research Fellowship.

This research fellowship is the first to be awarded as part of the new SNMMI Mars Shot Research Fund—established to provide resources that translate visionary nuclear medicine imaging, radiopharmaceutical therapy, and data science research or projects into tools or treatments helping improve the lives of patients.
Sharifi was awarded the $100,000 SNMMI/LBCA research fellowship based on her proposal, “Integrating minimally invasive biomarkers of estrogen signaling to detect endocrine therapy resistance in metastatic invasive lobular cancer.”
Despite recent therapeutic advances, metastatic breast cancer remains a leading cause of death in women in the U.S. due to treatment resistance. Lobular breast cancer, which accounts for about 15% of breast cancer diagnoses, is almost always estrogen driven (ER+) and treated according to standard approaches for ER+ ductal breast cancers. However, the investigation of lobular breast cancer cells in the lab has suggested that they are less likely to respond well to anti-estrogen therapy than ductal breast cancers, suggesting patients with metastatic lobular breast cancer would be more likely to experience anti-estrogen therapy resistance.
Unfortunately, there are not yet clinical diagnostic approaches for early detection of lobular tumors that are resistant to anti-estrogen therapy, and it is only after 3-6 months and endurance of drug side effects that patients with anti-estrogen resistant metastatic disease can be identified and offered alternative treatment approaches.
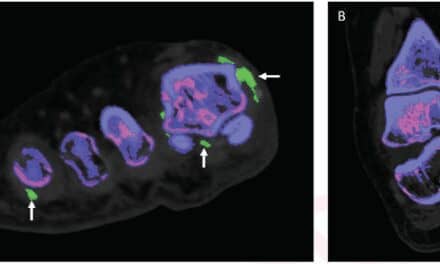
Sharifi intends to study an innovative approach that combines two non-invasive tests—one a blood test and the other an imaging test known as positron emission tomography, or PET, that uses an investigational radiopharmaceutical called F-FNP—to understand earlier whether treatment of the metastatic lobular tumor is effectively suppressing estrogen signaling and controlling or eradicating a patient’s lobular breast cancer cells. While both tests have been studied before in ER+ metastatic breast cancer, neither has been studied specifically in lobular breast cancer.
“The goals of this proposal are one, to identify how tumor estrogen signaling changes on treatment via the imaging test, two, to identify how tumor estrogen signaling changes on treatment via the blood test, and three, to understand whether the combination of the imaging and blood test can distinguish between patients whose cancers will respond to anti-estrogen therapy and patients whose cancers are resistant to this type of treatment.
This proposal would lay the groundwork for larger clinical trials testing the ability of this approach in identifying patients with anti-estrogen resistance early to offer them an alternative more effective treatment and minimize exposure to side effects from a treatment that is not effective. Early determination of drug sensitivity combined with accurately targeted therapies has the best potential to turn metastatic, lobular breast cancer into a manageable chronic, rather than deadly, disease.”