Running the Numbers
Angio Suites Add Tools for the Interventional Radiologist
Product Showcase: Mobile DR System Displays Images in 3 Seconds
GE OEC Medical Signs Consent Decree with FDA
Product Showcase: Image-Enhancement Software Analyzes Every Pixel
Running the Numbers
1% to 2% growth rate in US dollars is expected in the general US radiography market by 2011, according to the Global Markets for General Radiography Systems 2007 report from Millennium Research Group, Toronto. The report notes a similar moderate increase for the Japanese market; however, a 3% to 4% increase is expected for the European market. Also, the global analog market, currently valued at $330 million, will continue declining, and CR and DR systems will become more affordable. The report estimates that through 2011, CR will continue to be 65% to 75% less expensive than DR; the CR market will be valued at more than $1.7 billion. For more information, visit www.mrg.net.
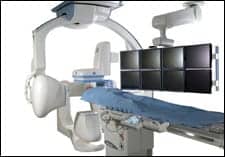
|
| The Innova 3131IQ digital flat-panel biplane imaging system from GE Healthcare covers the full size of the patient?s frontal and lateral anatomy simultaneously, enabling procedures to be performed with fewer x-ray images and fewer contrast injections. |
Angio Suites Add Tools for the Interventional Radiologist
Flat panels and portability were the buzzwords at RSNA 2006 as vendors displayed the latest advances in their angiographic x-ray technology.
The Innova 3131IQ and 2121IQ Biplane Imaging Systems from GE Healthcare, Waukesha, Wis?which were built based on input from dozens of clinical studies?feature proprietary digital flat-panel technology that enables high-quality 3D flat-panel rotational imaging of the vascular system, bone, and soft tissue. Offering 20- and 30-cm area coverage, the Innova IQ systems illuminate the finest vessel and cardiovascular technology while covering the full size of the patient?s lateral and frontal anatomy simultaneously.

|
| This angiographic 3D image of a high-grade stenosis of the left internal carotid artery in a 79-year-old male was acquired on the Siemens Axiom Artis dBA Twin system using syngo Inspace 3D and cross-sectional visualization by syngo DynaCT to evaluate stent deployment. Image courtesy of Siemens Medical Solutions and University Hospital of Erlangen-Nuremberg, Germany. |
Siemens Medical Solutions, Malvern, Pa, unveiled the largest biplane anatomical coverage area on the market?30 x 40 cm?on its Axiom Artis dBA Twin system. Featuring a compact housing design that enables virtually unrestricted patient access and outstanding angulation capabilities, the Axiom Artis dBA Twin offers flexibility across an array of applications. It also facilitates dose reduction for pediatric applications and supports the latest cross-sectioning 3D imaging techniques via the optional syngo DynaCT cross-sectional imaging capability.
Specializing in C-arm systems, Ziehm Imaging Inc, Riverside, Calif, displayed its Vision FD, Vision R, and Vario 3D devices, all of which were cleared by the FDA in 2006. The Vision R features a high-dynamic CCD camera running in pulse mode with up to 30 frames per second. The Vario 3D alternates between routine 2D fluoroscopy and isocentric or 3D mode with a simple keystroke; the full array of routine fluoroscopy applications is available on the device, making it a cost-efficient choice for hospitals where 3D is a necessity. And one of the first mobile C-arms to use flat-panel-detector technology, the Vision FD, is now cleared for use in the United States, moving the same CCD imaging capabilities provided by the Vision R from room to room with ease.
This feature is important in a market where patients are taking a growing interest in the price and quality of their care. “The informed patient understands why flat panel is preferable to analog,” explains Travis Sheridan, director of public relations at Ziehm. “The informed patient now understands the importance of using less radiation to capture a better image.”
? C. Vasko
Product Showcase: Mobile DR System Displays Images in 3 Seconds


|
| The Mobile DaRt from Shimadzu offers mobile DR with an easy-to-read LCD and storage of up to 2,000 images. |
Shimadzu Medical Systems, Torrance, Calif, recently introduced its MUX-100D Mobile DaRt digital radiographic mobile x-ray system. Mobile DaRt features a 14×17-inch digital flat-panel detector; at just 23 mm thick and weighing 4.8 kg, the detector covers a wide examination region without inhibiting the mobility of the DaRt. Three seconds after exposure, a reference image is displayed on the Mobile DaRt?s LCD?in other words, images can be validated immediately; image processing also can be conducted on the monitor. The device?s extra-long arm features a long stroke of up to 1,200 mm, facilitating difficult imaging situations. Also, a high-frequency inverter?operating at up to 60 kHz?helps the system generate low-ripple, stable x-rays for faster and more accurate radiography. Other features include simple network and printer output in DICOM format; storage of up to 2,000 images; intuitive maneuvering; “inch-mover” buttons on the front of the collimator for safe positioning; and clear display of x-ray timing via a bright, easy-to-read display. For more information, visit www.shimadzumed.com.
? C. Vasko
GE OEC Medical Signs Consent Decree with FDA
After the FDA made its most recent examination of General Electric Co?s Salt Lake City manufacturing facility, the agency issued a consent decree of permanent injunction prohibiting the manufacture and distribution of specified x-ray surgical imaging systems made by GE OEC Medical Systems?a division of GE Healthcare, Waukesha, Wis. The consent decree?filed on January 12?was signed by two of GE?s top executives and also applies to the company?s Lawrence, Mass, facility.
The Utah location?s manufacturing practices came under scrutiny during a November 2004 inspection. The FDA issued a Warning Letter on March 31, 2005, citing violations of its current good manufacturing practice (CGMP) requirements; the injunction followed when a 2006 investigation revealed inadequate responses to the letter. CGMP deficiencies cited included failure to establish and maintain adequate procedures for validating the device design, and failure to establish and maintain adequate procedures for implementing corrective and preventive actions.
“These devices are used on thousands of patients, and their dependability and accuracy are critical for the successful outcomes of important medical procedures,” Daniel Schultz, MD, director of the FDA?s Center for Devices and Radiological Health, said in a press release. “When the FDA?s August 2006 inspection found ongoing CGMP deficiencies at the Utah facility, GE voluntarily stopped distributing devices from that facility and is working with the FDA to ensure that necessary corrective actions are fully implemented.”
The devices affected include the 9900 Elite C-Arm System, the 9900 Elite NAV C-Arm System, the 9800 C-Arm System, the 2800 UroView System, the 6800 MiniView System, the Insta-Trak 3500 NAV System, and the ENTrak 2500 NAV System, as well as their components and accessories. Under the terms of the consent decree?signed by Joseph M. Hogan, president and CEO of GE Healthcare; and Peter McCabe, president and CEO of GE OEC Medical Systems?the companies will take necessary measures to ensure FDA compliance. The decree also requires that the companies hire an independent expert to conduct inspections of the Utah and Massachusetts facilities and certify to FDA that all required corrections have been made.
The consent decree allows the companies to continue to provide routine service, replacement parts, and accessories for existing x-ray surgical systems; however, the companies are required to submit to the FDA a corrective action plan for bringing into compliance the 9900 Elite C-Arm, the 9900 Elite NAV C-Arm, and the 9800 C-Arm systems that currently are in use in the United States.
GE OEC Medical Systems has initiated product recall on several models of its x-ray surgical imaging systems. Copies of the recall notices are available online at www.gehealthcare.com/usen/xr/surgery/oec_info.html.
? C. Vasko
Product Showcase: Image-Enhancement Software Analyzes Every Pixel

|
| Such details as the soft-tissue layers under the foot and visibility of the Achilles tendon are more visible in the GOPview XR2-enhanced image (above right) than in the standard x-ray (above left). |
ContextVision AB, Stockholm, Sweden, has released its latest image-enhancement system for digital x-ray. The GOPView XR2 uses adaptive technology, including a metal-safe algorithm, to optimize its noise-suppression, edge and contrast enhancement, and effective dynamic range compression capabilities. The system is modeled after ContextVision?s GOPView MR technology; the company also manufactures image-enhancement products for mammography and ultrasound.
“GOPView XR, for general radiography equipment, looks at each pixel in an image and analyzes what relation it has to each pixel around it,” explains Peter K?evamees, marketing manager for ContextVision. “What kind of structure does this pixel belong to? What kind of orientation does the structure have? When we have made a complete analysis of every pixel in the image, we can set a threshold for what will be determined as noise and what will be determined as structures. This is how we can separate the noise from the structures for much better visualization of the structures while suppressing the noise.”
One of the major enhancements of GOPView XR2 is an advanced algorithm for improved clarity in areas with metal implants. “It provides the sharpest orthopedic imaging,” K?evamees says. “We have implemented a metal-safe algorithm, meaning we don?t get any white and black areas close to metal implants.” He notes, “It?s very fast, and it could enable dose reduction. We are doing some clinical evaluations; we have not finalized them, but we do expect high dose-reduction rates.” He says that the XR2 also helps with artifacts: “We take care of artifacts by feedbacking information from a higher to a lower hierarchical level when we do the calculations. ? When you lower the number of photons that goes through the organ, you get a noisier image. Our algorithms are expert at finding and eliminating noise. By lowering the dose and using our algorithms to lower the noise, you can get the same image quality as you would with the full dose.”
Visit www.contextvision.se for details.
? C. Vasko




